Winter rain is lighter than that in summer. Does not make sense? Yes, it does! The cause are stable water isotopes.
Water is made up of 2 types of atoms: Hydrogen (H) and Oxygen (O). These Atoms come in different varietys, also called isotopes. The isotopes of water exist in two weight levels: light 1H and heavy 2H as well as light 16O and heavy 18O.
During evaporation, light water (made of 1H and 16O) is preferentially absorbed by the air. Depending on the temperature, more or less molecules of light water condense in the air and rain out. When it is warm, there are significantly fewer light atoms in the rain than when it is cold.
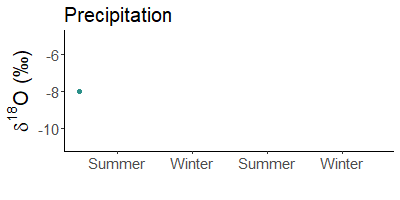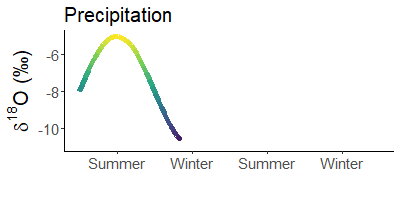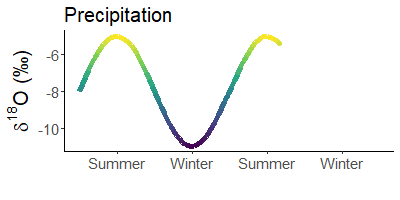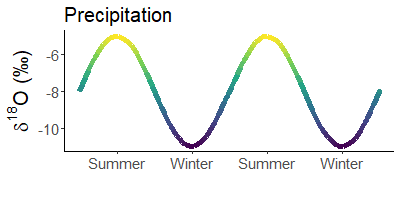
Since it is much colder in winter than in summer, precipitation is much heavier in summer than in winter. For example, snow is often the lightest precipitation of the year.
In isotopie hydrology, the delta notation (e.g. δ2H or δ18O) is used to describe the ratio of heavy and light water in relation to a standard.


This post is part of my water science communication series #waterwednesday where I weekly post short researchfindings or just stuff that interests me
Sources:
Rozanski, K., Araguás-Araguás, L. and Gonfiantini, R. (2013) “Isotopic patterns in modern global precipitation,” Climate Change in Continental Isotopic Records, pp. 1–36. Available at: https://doi.org/10.1029/gm078p0001.


Comments are closed